You are invited to take part in a study to investigate a new vaccine for Crimean-Congo haemorrhagic fever virus. The study is being led by the Oxford Vaccine Group, which is part of the University of Oxford, and is taking place at the Centre for Clinical Vaccinology and Tropical Medicine at the Churchill Hospital.
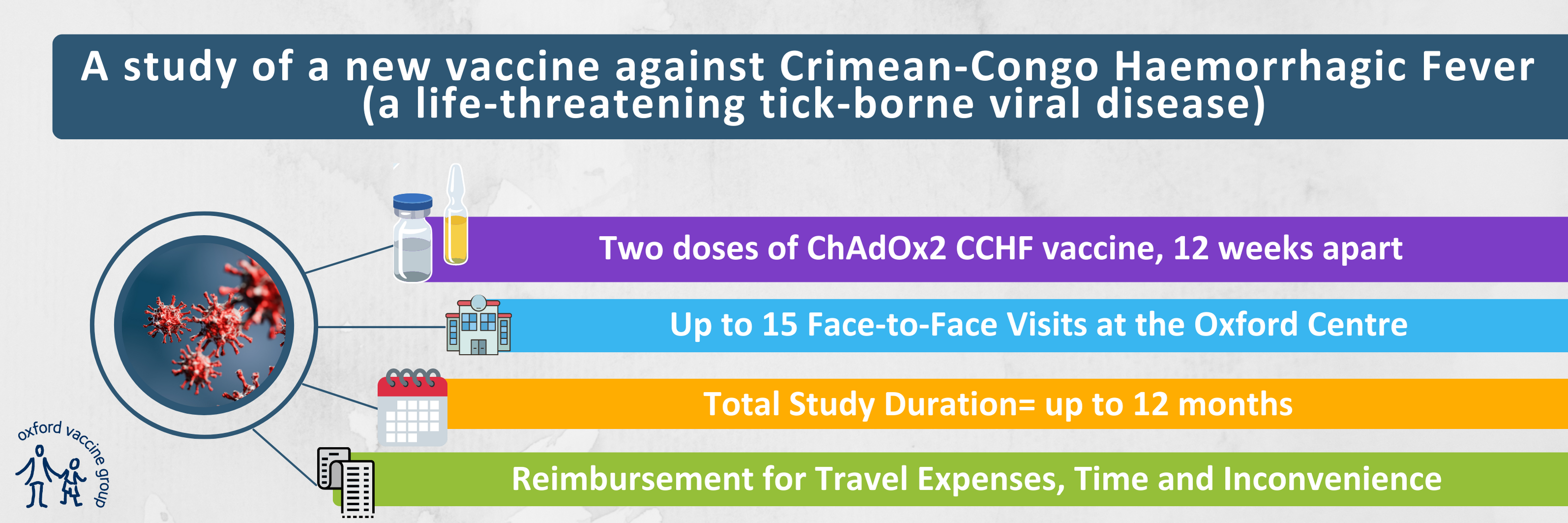
If you are 18 to 55 years old, in good health and live in the Oxfordshire area, then you may be eligible to take part. We will provide reimbursement between £1,110 and £1,470, for your time, inconvenience and travel. The total study participation time is 12 months.
Background
Crimean-Congo haemorrhagic fever (CCHF) is caused by a virus which is spread by ticks.
These live on a wide variety of wild and domestic animals, including cattle, sheep and goats.
People usually acquire the infection after being bitten by a tick, but the disease can also be caught directly from infected animals or people.
The infection does not always cause illness; but, in some cases, it causes very serious disease, often with impaired blood clotting, which can lead to serious bleeding. Up to 40 percent of people admitted to hospital with the disease will die. There are no proven, specific treatments for the disease, and no approved vaccines.
Crimean-Congo haemorrhagic fever occurs over a very wide geographic area, including parts of Southern Europe, the Middle East, Africa and south-west Asia. The World Health Organisation estimates that about 3 billion people live in areas at risk of the disease. Ten to fifteen thousand people are infected by the virus each year, of whom about 500 die as a consequence.
Study Goals
The University of Oxford has developed a vaccine against Crimean-Congo haemorrhagic fever called “ChAdOx2 CCHF”. This study will be the first time this vaccine is given to humans.
We are aiming to confirm the vaccine’s safety and investigate the development of immunity following vaccination.
For further information, please contact us to request the Participant Information Sheet or to speak to one of the study team.
Study Details
If you are eligible and decide to take part, you will receive two doses of the study vaccine, given 12 weeks apart. After receiving each dose of vaccine, you will be required to complete an online diary for 28 days. You will have blood tests to assess your immune response to the vaccine.
The Oxford Vaccine Group are based at the Centre for Clinical Vaccinology and Tropical Medicine at the Churchill Hospital, where all study visits/appointments will take place.
Further Information
If you would like to find out more, please read the Participant Information Sheet PIS
In addition, if you would like any further information regarding the study please contact us on:
Email: info@ovg.ox.ac.uk Tel: 01865 611400
Kind Regards,
The Oxford Vaccine Group
